The pH of your skin can influence your acne in many ways. It also has profound effects on the stability of your skin and balance of skin bugs.
Do you know the pH of your face wash? Probably not, because most products do not disclose it!
Of many factors that influence skin health, skin pH rarely draws attention as people are more concerned about the effects of diet, smoking, drinking, and exercise. When buying a skincare product, we hardly attempt to read the label. In fact, we are easily carried away by the marketing taglines.
All things considered, it’s time to learn the effects of pH on skin health and how skincare products influence pH.
The Basics: What is Skin pH and the Acid Mantle?
The pH, or the potential of hydrogen, of a substance gives an idea of how acidic or basic it is. The scale ranges from 0 to 14. Simply put, an acidic substance has a pH less than 7 and a basic or alkaline substance has a pH greater than 7.
Distilled water is neutral with a pH of 7, whereas the soap is generally alkaline with a pH between 9 and 11. The pH of normal healthy skin ranges from 5.4 to 5.9, which means that healthy skin is slightly acidic.

You might see the term “acid mantle” used in many articles. The acid mantle refers to the pH of the outermost layer of the skin. This outermost layer is called the stratum corneum and has the following functions:
- Strengthening the skin’s barrier, which is crucial for preventing excessive loss of water from the skin and limiting the entry of bacteria and other foreign substances into the skin.
- Hastening the recovery of the barrier, in case it gets damaged.
- Improving skin integrity and making it more resistant to everyday contact.
- Shedding of skin cells, which can help with wrinkles and age spots.
- Creating an acidic environment that prevents the growth of harmful skin bugs.
Why Skin pH is Acidic
The pH of healthy skin is acidic for several reasons:
- Sweat glands: Sweat glands produce sweat, which is about 80 percent sodium chloride and 11 percent lactate. Lactate is responsible for causing a drop in the skin pH.
- Free fatty acids: Free fatty acids are produced from the breakdown of fat molecules on the skin and make the skin more acidic.
- Sebaceous glands: These glands produce an oily substance (sebum) that helps keep the skin lubricated. Increased sebum production can cause acid pH by increasing the availability of free fatty acids. However, in acne, it is seen that sebaceous glands do not increase FFA availability. It could be one of the reasons why oily skin further aggravates acne, apart from clogging the pores.
- Na/H antiporter: This is a proton pump that increases the concentration of hydrogen ions, which in turn decreases the pH of the skin.
Top Effects of pH on Skin Health
pH has widespread effects on skin integrity, flora, hydration, and permeability. Here are some of the most significant effects of pH on skin health, according to scientific studies.
- Having a higher facial pH can increase the risk of acne or its recurrence.
- Keeping skin pH in the range of 4 to 4.5 helps maintain normal skin flora and protect the skin against bacteria. On the other hand, an alkaline pH tends to disrupt it.
- Applying acidic skin products can improve barrier function and reduce surface roughness. This was shown in a 2018 study of older adults who used a topical formulation with pH of 4 for 4 weeks.
- Alkaline skin pH is associated with low skin hydration, moisture loss, and low lipid levels on the skin.
pH Levels in Common Skin Problems
According to the American Academy of Dermatology, the top 3 skin conditions in the U.S. (with the exception of hair loss) include acne, atopic dermatitis (eczema), and psoriasis.
Let’s take a look at the pH levels associated with these conditions and the role of pH in the disease causation or progression.
Acne and Skin pH
Acne is estimated to affect up to 50 million Americans, most of whom are under the age of 25. Given that acne is the most common skin condition in the U.S., it is important to learn the role of skin pH in acne.
Acne-prone skin has an alkaline pH, which predisposes people to acne in several ways:
- Impaired barrier function of the stratum corneum (outer skin layer), making it more vulnerable to acne-causing bacteria
- Decreased activity of dermicidin, a natural bacteria-fighting substance that works best at pH of 5.5. When skin pH increases, dermicidin become less effective and the activity of acne-causing bacteria increases.
- Reduced production of free fatty acids from sebaceous glands. Studies have found that acne-prone skin has lower levels of free fatty acids. This is yet another reminder that free fatty acids are necessary for ideal skin pH.
Eczema and Skin pH
Eczema is the second most common skin condition, affecting 28 million Americans. In children with eczema, the affected skin has a higher pH compared to unaffected skin. Furthermore, the overall skin pH of those with eczema is higher than that in people without eczema.
Psoriasis and Skin pH
Psoriasis affects 7.5 million people in the United States. Up to 90 percent of these people have plaque psoriasis, which causes patches of raised, reddish skin.
It is well known that altered skin pH results in impaired barrier function, which promotes inflammation and aggravates existing psoriasis. Not surprisingly, several studies have observed increased skin pH in patients with psoriasis.
The Role of Skin Treatments on pH
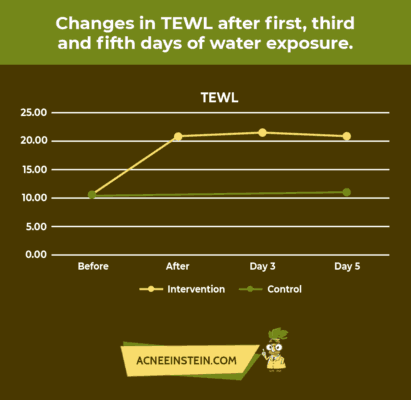
Did you know that washing your face with water can increase its pH? Now, can you imagine what soap could do to your skin? Horrible changes can happen to your skin if you use the wrong products.
A recent study concluded that after 5 consecutive days of daily water exposure, the skin pH increased. The amount of moisture loss, measured by total epidermal water loss (TEWL), also increased with daily water exposure to the skin. These results are shown in the following graphs.

Unfortunately, most skincare products do not mention pH on the label. This can be tricky as consumers are easily misguided by clever marketing tactics.
Most notably, two different products of identical cleansers showed significantly different effects on the skin due to their pH levels (5.5 versus 7). At the end of the study, the more alkaline product resulted in higher skin pH and prevalence of acne-causing bacteria. It’s clear that even minor pH changes in skin products can alter skin pH and hence increase the likelihood of developing acne.
A recent study even predicted certain products to be detrimental for skin health due to their effects on skin pH and hydration. However, it might be too early to jump to any conclusions since this study only used mice models. Another study observed favorable results with a cleanser specifically formulated for normal to oily skin in people with mild facial acne.
Therefore, it’s important to keep in mind that a specific product can have both favorable and detrimental effects on the skin depending on your skin type and genetic makeup. Nonetheless, choosing an acidic product with a lower pH could be the best choice you can make for your skin.
Synthetic detergents, often referred to as syndets, are perhaps a better option than soap. With a pH of about 5.5, syndets are not alkaline and do not disrupt the balance of microorganisms on the skin. Such products may be beneficial for cleansing both healthy and diseased skin.
How to Restore pH Balance to the Skin
Using a skincare product with low pH is one of the most effective ways to restore healthy pH balance to the skin. Consider using a product that contains one of these acidic ingredients: alpha-hydroxy acids (i.e. lactic acid, glycolic acid), retinol, ascorbic acid (form of vitamin C), or kojic acid.

But remember to also look out for other ingredients that can potentially damage the skin barrier or cause irritation, such as sodium lauryl sulfate (SLS), alcohol, or strong fragrance. In particular, SLS is a common staple of numerous cosmetic products with rising concerns over its irritant effects on the skin.
Given these considerations, here are some options to help maintain a healthy skin pH:
Toner
Toner is a liquid that helps re-acidify the skin after cleansing. In addition, it also clears impurities, removes dead cells, and promotes absorption of other skincare products applied after cleansing (i.e. moisturizer).
If you wash your face with a high-pH cleanser like soap, using a toner can help restore normal pH. However, this may not be necessary if you already use a low-pH cleanser.
Micellar Water
Micellar water is often promoted as an alternative to harsh cleaners, but does it actually work? Let’s dive into how this works.
Micellar water is a clear, water-like solution with a mild surface-active agent (or simply “surfactant”). When applied over the skin, the surfactant molecules form sphere-like structures called micelles, hence the name “micellar” water. The inside of the micelle encloses the oily dirt while the outer surface is water-soluble and can be wiped away with a cotton swab.
This same phenomenon occurs with face cleanser and laundry detergent. That being said, micellar water and face cleanser are not much different. The only possible variance could be that micellar water only uses a mild surfactant, but the same effects can be observed with mild cleanser.
If you’re not in a mood to spend your hard-earned money on such fancy items, however, there is another option. You can make your own acidic cleanser by using apple cider vinegar (ACV), which contains citric and malic acids.
DIY Acidifying Water with Apple Cider Vinegar
To make your own acidic cleanser with ACV:
- Add ½ teaspoon of vinegar to at least 16 oz of water.
- Stir the solution properly.
- Optional: add rose water to it for fragrance.
Note that using ACV-water on the skin only reduces the skin pH but does not clear the oily dirt from your face. Moreover, using the acidic water may not be suitable for those with sensitive skin.
For those who cannot stomach the scent of ACV, below is another easy recipe with lemon juice.
DIY Acidifying Water with Lemon Juice
Lemon is another great choice for acidifying water. Since lemon juice is highly acidic with an estimated pH between 2 and 3, it is not recommended to use undiluted lemon juice. But no worries! You can dilute it with water to raise the pH.
To make your own acidic cleanser with lemon juice:
- Add 1 mL of the juice to 1000 mL of water for a final pH of 5.
- Stir the solution properly
Note that lemon can make your skin sensitive to sunlight. Therefore, make sure to use a sunblock while using this remedy. In addition, you should probably avoid this option if you have sensitive skin or use acne medications such as topical retinoids.
pH-Balanced Cleansers
pH-balanced cleansers are another great way to keep the skin pH in healthy range. But the big question is, what is the optimal pH for a cleanser? Based on a few studies, we suggest using a soap-free cleanser with a pH of about 5.5 for both healthy patients and those experiencing skin problems. This corroborates the PubMed Health recommendation for people with acne.

However, the problem with cosmetic products is that they usually do not mention pH on their labels. They usually mention the percentage of the active compound instead. Based on the FDA guidance for cosmetic labeling, we can say that a formulation with 10% or less “acidifying substance” is generally safe, so long as you are using daily sun protection.
Here is some insight about the pH levels of popular skincare products:
- Ninja Skincare Revolutionary Facial Cleanser: acidic, but exact pH unknown
- Yes to Carrots Daily Cream Facial Cleanser: pH ~ 6.0 to 7.0
- Rodan and Fields Unblemish Sulfur Acne Wash: pH ~ 8.5
Our Product Recommendations
Based on what we discussed in this post, here are some of the best acidic cleansers we recommend for skin care:
- Phace Bioactive Detoxifying Cleanser: This amazing cleanser boasts a super-low pH of 3.0 to 3.5. That being said, it is gentle on your skin and also contains the goodness of two key natural plant extracts bisabolol and Centella asiatica. Don’t miss it especially if you have oily skin.
- CosRX Low pH Good Morning Gel Cleanser: This gentle face cleanser has a favorable pH, which is between 5.0 and 6.0. In addition, tea tree helps relieve acne.
Take-Away Message
As you can see, the acidity of your products can play a huge role in your skin health. By keeping your skin slightly acidic, you can keep your skin replenished and significantly reduce your risk for problems like acne and eczema. Unfortunately, many cosmetic labels do not disclose the exact pH of their products.
To maintain a balanced skin pH, we recommend using toners, micellar water, and soap-free facial cleansers with 10% or less acidifying ingredients (i.e. lactic acid, glycolic acid, retinol, ascorbic acid). Don’t forget to use proper sun protection and be extra cautious if you have sensitive skin! While skincare products may contain favorable pH properties, they may also contain irritation-causing substances such as sodium lauryl sulfate.
What has your experience been with acidifying water and cleansers? Feel free to share your insight about this topic – we’d love to hear from you!




